Lunai Bioworks Identifies Three Parkinson's Subtypes and Prioritized Drug Targets to Accelerate Proof-of-Concept Programs and Strategic Partnerships in a $13B Market
SACRAMENTO, Calif., Dec. 9, 2025 /PRNewswire/ -- Lunai Bioworks (NASDAQ:LNAI), an AI-powered biotechnology company developing precision therapeutics, today announced the identification of three clinically relevant Parkinson's disease subtypes and prioritized drug targets that may accelerate proof-of-concept programs, derisk therapeutic development, and enable strategic co-development partnerships in a growing $13 billion market.
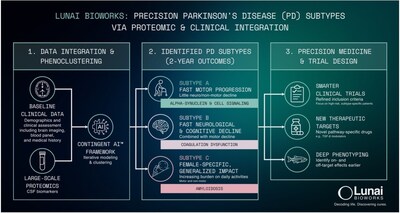
Using its proprietary Augusta Platform, Lunai Bioworks' wholly owned subsidiary BioSymetrics integrated large-scale proteomic data from the Parkinson's Progression Markers Initiative (PPMI), a landmark longitudinal study managed by The Michael J. Fox Foundation that tracks thousands of patients over time to identify biological markers of disease progression, with high-resolution clinical phenotyping to uncover subtypes associated with rapid progression, cognitive decline, and functional impairment. This analysis was recently highlighted in a recent article in Science Times.
The analysis integrated longitudinal clinical and proteomic data from more than 650 participants across 4,500 proteomic probes, tracked over multiple years (median ≥2.5 years, many ≥5 years). This scale enabled statistically robust identification of molecular signatures linked to rapid progression and worse outcomes, supporting biomarker-enriched patient selection, accelerated proof-of-concept, and higher probability of clinical and commercial success for subtype-specific therapeutics.
Subject-level analyses revealed three outcome-linked patient subtypes:
- Fast motor progression with limited non-motor involvement
- Rapid neurological and cognitive decline alongside motor worsening
- A female-enriched subtype with broad functional impairment
These findings create an actionable foundation for inclusion, enrichment, and endpoint strategies designed to improve trial success rates, time to proof-of-concept, and asset valuation.
Proteomic and pathway-level evaluation identified progression-linked targets and biomarker candidates that may support baseline stratification, progression monitoring, and treatment-response assessment. Lunai is initiating experimental validation of prioritized targets and advancing toward preclinical model development and biomarker qualification.
"As Parkinson's therapy development continues to struggle with high failure rates and slow progression signals, subtype-specific strategies can materially improve outcomes," said David Weinstein, CEO of Lunai Bioworks. "By linking clinical trajectories to biological pathways, we can design smarter trials, identify partnerable targets, and accelerate development timelines in a highly competitive market."
In parallel, Lunai Bioworks is evaluating co-development and partnering opportunities with biopharmaceutical companies to:
- Apply subtype-specific inclusion criteria to existing Parkinson's assets
- Co-develop biomarkers and companion diagnostics
- Translate pathway-level insights into first-in-class therapeutics
"Integrating molecular biology with clinical phenotyping gives us a mechanism for identifying precision targets that could reshape how Parkinson's therapies are developed," said Dr. Gabe Musso, Chief Scientific Officer of BioSymetrics. "This will enable faster validation and more efficient partnering strategies."
Parkinson's disease represents a $6-8 billion market today, projected to exceed $13 billion by the 2030s, driven by rising prevalence and unmet need. Lunai Bioworks believes that by integrating phenomics, proteomics, and precision stratification, it can increase clinical success rates, shorten timelines, and support high-value partnerships.
About Lunai Bioworks
Lunai Bioworks Inc. (NASDAQ:LNAI) is an AI-powered drug discovery and biodefense company pioneering safe and responsible generative biology. With proprietary neurotoxicity datasets, advanced machine learning, and a focus on dual-use risk management, Lunai Bioworks aims to redefine how artificial intelligence accelerates therapeutic innovation while safeguarding society from emerging threats. For more information, please visit: https://lunaibioworks.com.
Forward-Looking Statements
This press release contains forward-looking statements, including statements regarding potential clinical impact, therapeutic benefit, development timelines, partnering strategy, and commercial value. These statements are subject to risks and uncertainties that may cause actual results to differ materially. Lunai Bioworks undertakes no obligation to update forward-looking statements, except as required by law.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/lunai-bioworks-identifies-three-parkinsons-subtypes-and-prioritized-drug-targets-to-accelerate-proof-of-concept-programs-and-strategic-partnerships-in-a-13b-market-302636013.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/lunai-bioworks-identifies-three-parkinsons-subtypes-and-prioritized-drug-targets-to-accelerate-proof-of-concept-programs-and-strategic-partnerships-in-a-13b-market-302636013.html
SOURCE Lunai Bioworks Inc.